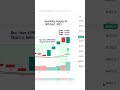
Sharemasterindia.com: Aurobindo Pharma shares surged over 4 per cent to hit 52-week high of Rs 1,454 on the National Stock Exchange.
Including today's gain the stock has surged around 14 per cent in last seven trading sessions after the company said that it had received final approval from the US Food & Drug Administration (US FDA) to manufacture and market extended Phenytoin sodium capsules, which is an anti-epileptic drug used for controlling seizures.
Aurobindo Pharma has also received final approvals from the US FDA to manufacture and market Entacapone Tablets, used in the treatment of Parkinson's disease having an estimated market size of $59 million for the twelve months ending April 2015 according to IMS, the company said in a filing on June 22.
In another filing on June 22, the company said that it has got final approvals from the US FDA to manufacture and market Azithromycin for injection USP, a macrolide antibacterial drug indicated for the treatment of patients with infections by susceptible strains of the designated microorganisms in the conditions such as Community-Acquired Pneumonia and Pelvic Inflammatory Disease, the company said.
Post these approvals Aurobindo Pharma has now 12 ANDAs approved out of formulation facility in Hyderabad. Analysts say the strong pipeline of new drug approvals will boost its future revenues.
As of 1:00 p.m. Aurobindo Pharma shares traded 3.80 per cent higher at Rs 1,449.05 apiece, outperforming the broader Nifty, which was up 0.34 per cent.